Our task was to determine the polarity of the molecules we did ball-and-stick diagrams for in yesterday's lesson.
Here are a couple of observations we need to be able to explain, using molecular polarity:
- Polar solutes dissolve better in polar solvents. Non-polar solutes are only sparingly soluble in polar solvents. Why? The example we were given was ammonia (NH3) dissolving in water.
SOURCE: UC Davis ChemWiki - Polar molecules have higher melting and boiling points than non-polar molecules with a similar-sized electron cloud. For example, methane (MR = 16.0 gmol-1) is a gas at room temperature while water (MR = 18.0 gmol-1) is a liquid. Why?
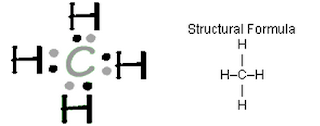
SOURCE: http://chemsite.lsrhs.net/bonding/lewis_dots.html
 |
| SOURCE: http://www.ausetute.com.au/lewisstr.html |




No comments:
Post a Comment